Newfront Clinical Trial Portal Unveils Enhanced Brand Interface and Feature Set
By Newfront | Published December 6, 2023
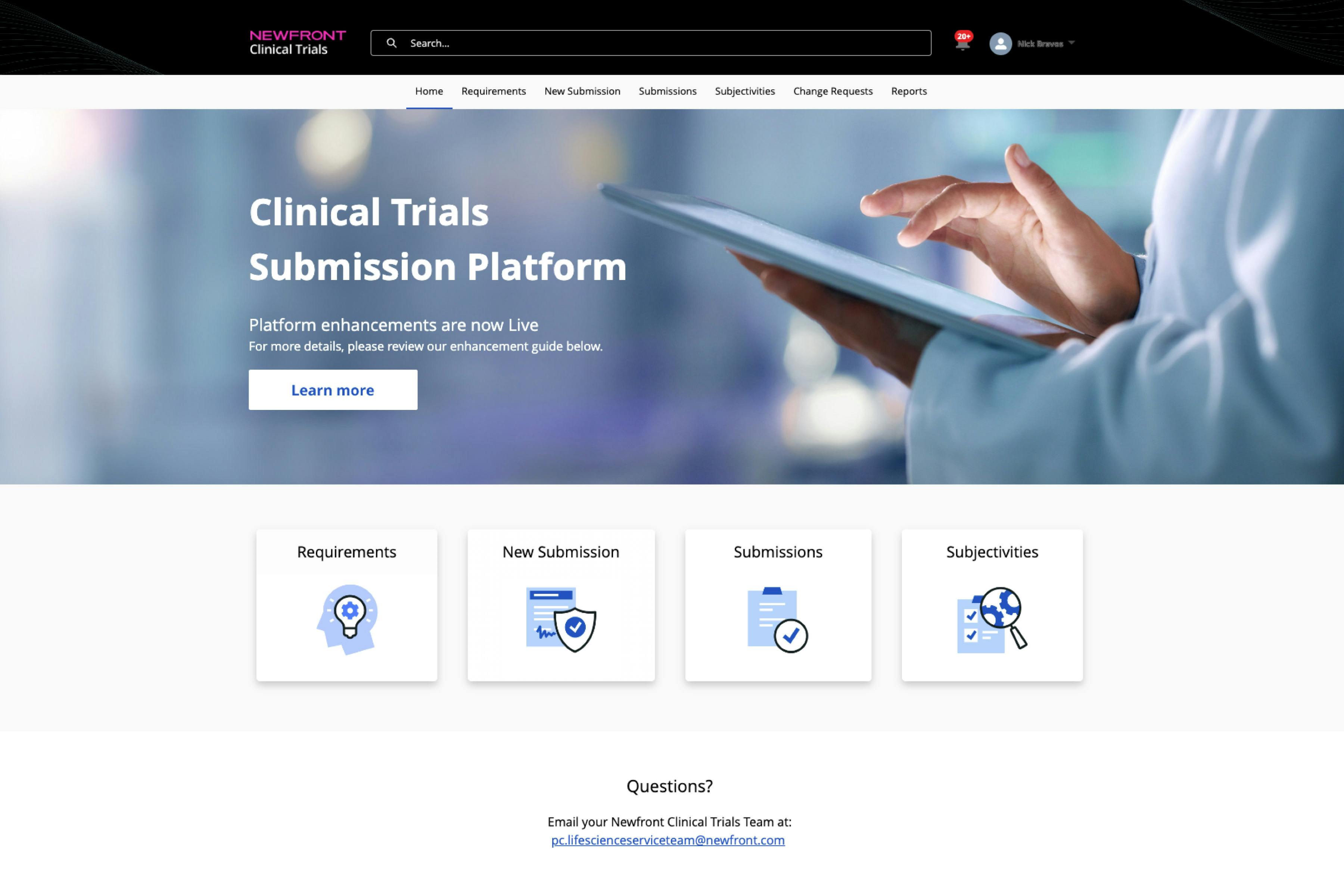
Newfront's breakthrough Clinical Trial Portal has been upgraded, with a refreshed brand interface and a range of feature enhancements, including a streamlined request process, more flexible trial management, and better document organization.
Any clinical trial manager will tell you: managing clinical trials and the insurance policies that enable them can be a complex and time-consuming process. One of the most game-changing innovations in the industry in recent years has been the Newfront Clinical Trial Portal. Created based on feedback from Life Science clients conducting clinical trials, the portal is a 24/7 platform that lets trial managers seamlessly manage their programs, including accessing country requirements, securely uploading documentation, requesting quotes and receiving terms in a timely manner, and generally facilitating easier communication between clients, brokers, and carriers. After a significant upgrade earlier in 2023, we have now added an upgraded brand interface and a range of enhanced features.
New Brand Interface and Refreshed Video Guide
The Newfront Clinical Trial Portal has undergone a complete makeover, presenting clients with a modern and intuitive brand interface that aligns with Newfront’s all-new brand identity launched in July. The refreshed design ensures a simplified user experience, enhanced accessibility and ease of navigation. Alongside the new interface, a revamped instructional video guide will soon be available to help clients quickly familiarize themselves with the portal's functionalities, ensuring a smooth onboarding experience.
Streamlined Requests and Document Organization
To expedite the quote process, the portal's request form has been updated to streamline the data we request from clients. We’ve also added a general queries option. Clients can easily submit queries or seek clarifications directly through the portal, enhancing communication channels and fostering efficient collaboration. Finding documents is easier, too, as documents are now conveniently organized and identified by type, enabling quick access to important files when needed.
Greater Flexibility in Policy and Trial Management
The updated portal also makes it easier for clients to modify their trials. A new section clearly defines how and where to lodge change requests, including adding trial sites, increasing patient counts, modifying expiry dates to extend study timelines , changing study titles, and making general inquiries. Change requests are sent through the portal to the Life Science service team for handling. These updates make it easier to manage trials as they expand and ensure adequate coverage as trial populations grow.
Better Trials, Better Coverage
The Newfront Clinical Trial Portal's brand interface refresh and introduction of enhanced features mark another milestone in the management of clinical trial insurance. With a streamlined navigation, refreshed video guide, improved request functionality, and additional capabilities the portal empowers Newfront clients to seamlessly navigate the complexities of insurance management. Clinical trial stakeholders can devote more time to advancing medical breakthroughs while confidently managing their insurance needs.

Newfront
Newfront is a modern brokerage transforming the risk management, business insurance, total rewards, and retirement services space through the combination of elite expertise and cutting-edge technology. Specializing in more than 20 industries and headquartered in San Francisco, Newfront has offices nationwide and is home to more than 800 employees serving organizations across the United States and globally.
Related Articles

Casualty 2024 Outlook: Emerging Risks and Ongoing Coverage Concerns
April 12 • 2024

Pharma's Almanac: In what areas of pharma/biopharma are you most excited to see increased adoption of artificial intelligence (AI) and machine learning?
August 8 • 2023

Event Recap: Insuring Life Sciences—Smart Strategies for Legal Leaders
July 24 • 2023